In recent months, MME has been approached by many clients seeking to understand the impact of COVID-19. Through our research and ongoing discussions, we’ve identified the wide range of new, large, and complex challenges facing payers, providers, and patients in these unprecedented times. The trends we’ve uncovered have been outlined in our research reports.
Now, we’ve turned our attention to understanding how COVID-19 will likely affect orphan therapies. Payers are facing issues from a pandemic, which arguably falls on the opposite end of the epidemiological scale from rare diseases and orphan drugs. We’ve summarized the key findings below:
- Less focus with limited time—Payers report prioritizing industry meetings with new drugs as well as drugs with a high PMPM impact. While the former is certainly applicable to orphans, the latter is less certain. We expect the short-term focus on orphan/rare meds might be limited to only proper diagnosis and administration.
- Lower future budgets—Some payers foresee previous challenges being augmented with the economic downturn and, as a result, orphan budgets could recover once the crisis abates.
- In the US, short-term increases in uninsured patients could significantly impact implications on gross-to-net assumptions, especially related to mandatory Medicaid rebates.
- Reprioritization/competition for attention—Despite a likely expansion of health budgets in some EU countries, rare diseases may have to compete for attention due to the expected reprioritization of budgets across healthcare systems. For instance, despite the expanded focus on local manufacturing of critical therapies to ensure availability, prioritization is almost certain to be given to larger disease categories over orphan/rare meds.
- More helpful communication—COVID-19 has exposed the need for systems to be better connected, which includes communicating about patient care to more effectively help rare disease patients. For example, expanding the use tele-medicine could significantly shift modes of care in the future.
- Compliance—Once a decision to cover a drug is made, some of the biggest waste perceived by payers are from non-compliance. There is an opportunity to align interests with compliance solutions.
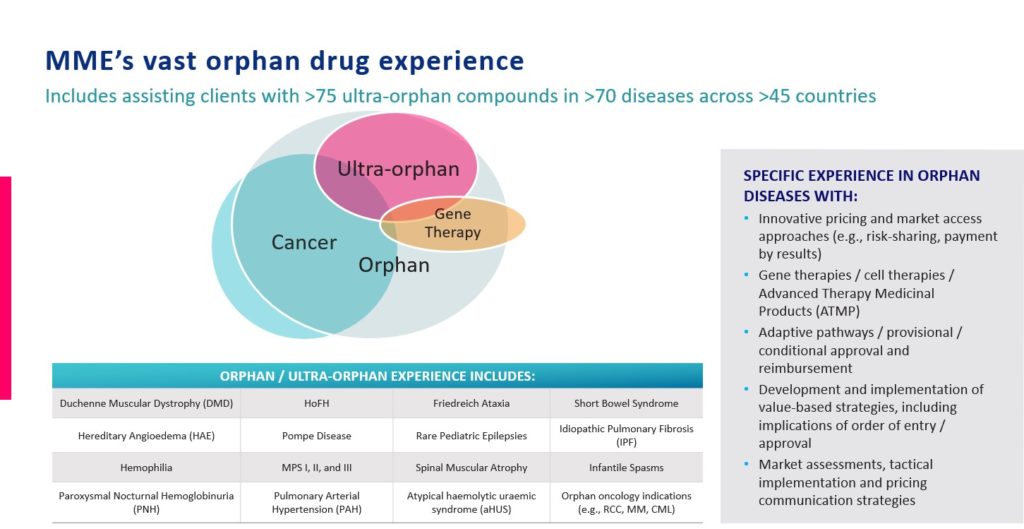
Overall, while increased pressures on healthcare systems from COVID-19 manifest in different ways, how they will impact different patients, diseases, and therapies will not be the same. Within the orphan and rare sectors, these pressures will provide short, intermediate, and long-term opportunities and threats for both marketed products and those in development. Contact us so we can talk about issues you may be facing and the implications for your future.